Functional Peptides
So far, the term "functional peptides" has
been used only for bioactive peptides such as suppliments and drugs.
From completely different viewpoints, we consider the peptides as
functional organic molecules, and have developed peptide catalysts,
peptide assemblies as nano vessel and template. Their
intra/intermolecular interaction is robust and many of them
successfully work under aqueous conditions.
1.
Peptide catalysts
Peptide catalyzed regio- and enantioselective addition to a,b,g,d-unsaturated
aldehydes (Angew. Chem. Int. Ed., 2013, 52,
11585)

Construction
of all-carbon substituted quartarnary chiral center via
peptide-catalyzed conjugate addition (Angew. Chem. Int. Ed., 2012,
51, 12786)

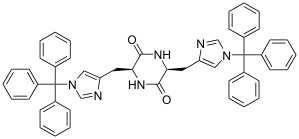
Catalytic
asymmetric Diels-Alder reaction using cyclic dipeptide as a ligand.
(Heterocycles,
2009, 78, 1171
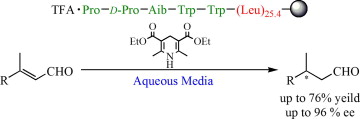
Asymmetric
hydrogen transfer reaction in aqueous media catalyzed by
resin-supported peptide catalyst having polyleucine chain.(Org.
Lett., 2008, 10, 2035; Tetrahedron:
Asymmetry, 2009, 20, 461)
One-pot
sequential acid- and base-catalyzed reactions including
enantioselective step.(Tetrahedron Lett. 2007,
48, 985-987)
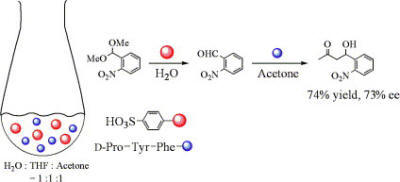
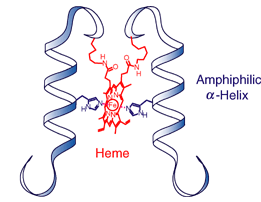
Control of the
reactivity of peptide-heme complex due to the structural change
induced by solvent.(Chem. Lett. 2006, 35,
584)
Asymmetric
cross aldol reaction in aqueous media catalyzed by resin supported
peptides.(Tetrahedron Lett. 2005, 46,
8185)
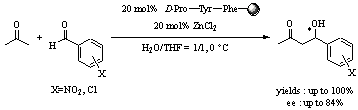
2. Peptides
as templates
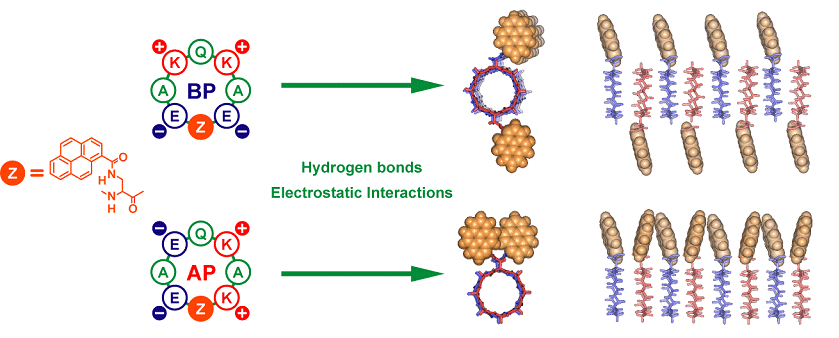
Orientation
control of self-stacking D,L-alternating cyclic octa-α-peptide
through multiple electrostatic interactions (Chem. Lett.,
2007, 36, 1070)
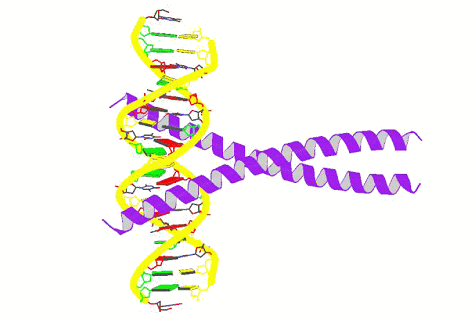
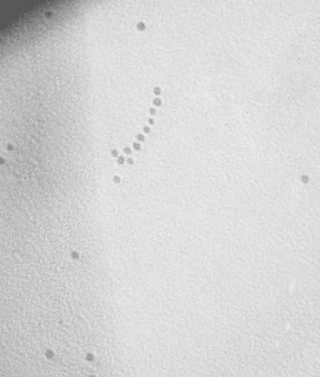
Preparation of
1D-aligned gold nanoparticles on DNA utilizing DNA-binding peptides
( Seisan-kenkyu (in Japanese) 2007, 59,
128)