
Project Professor Yoneda takes a step closer to producing a much more effective COVID-19 vaccine available with a groundbreaking approach.
Since the global outbreak of COVID-19 in 2020, hundreds of drug makers have been racing against time to make safe, effective vaccines available to stop the pandemic, which has claimed over 2 million lives. While most of the vaccines being administered or tested are inactivated, Project Professor Misako Yoneda, an applied veterinary science specialist at UTokyo-IIS, is taking a unique approach: developing a live, recombinant vaccine by using the measles virus as a vector.
While conducting experiments a few years ago, Yoneda realized how effective a recombinant measles virus was in preventing Nipah virus infection, a zoonotic illness found mostly in Bangladesh and India. As the COVID-19 pandemic raged in 2020, Yoneda decided to apply genetic engineering technology that uses the measles virus as a vector to develop a live vaccine against COVID-19.
In September 2020, Yoneda's research team started experiments on engineering a suitable virus for the vaccine at a high-level biosafety lab with a grant from the Japan Agency for Medical Research and Development. Within a couple of months, the team succeeded in synthesizing a recombinant measles virus expressing S protein, a COVID-19 antigen (the pathogen we are trying to vaccine against). Experiments using hamsters have proven the recombinant measles virus' effectiveness in preventing COVID-19 infection. Production of an actual vaccine using the virus is scheduled to begin soon, with the aim of starting animal testing in 2021 and Phase-1 clinical trials by early 2022.
"A live vaccine can induce virtually lifelong immunity against COVID-19," Yoneda said. "So, people don't have to get vaccinated regularly - say once every six months or every year - as required to maintain immunity with inactivated vaccines."
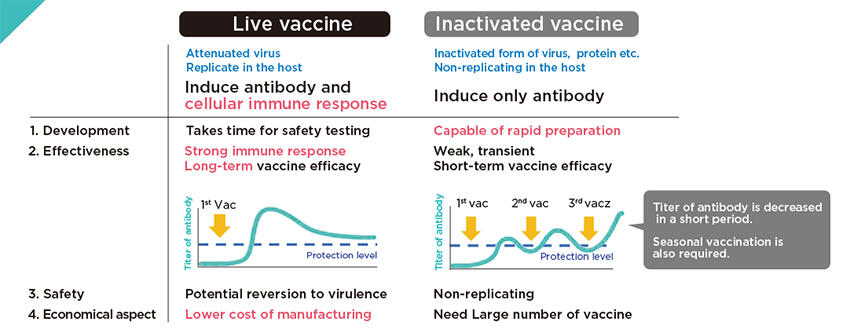
Live vaccines, which use a version of the living virus that has been weakened, are known to induce both antibodies (blood proteins produced in response to an antigen) and cell-mediated immunity, which can provide long-lasting protection against an antigen. Cell-mediated immunity is an immune response in which certain cells are mobilized to destroy antigens in the infected cells. So far, the only live COVID-19 vaccine authorized for use is an adenovirus-vectored vaccine, which Yoneda said is safer, but less effective, than the vaccine she is developing.
On the other hand, inactivated vaccines induce only antibodies, resulting in weak and temporary effects.
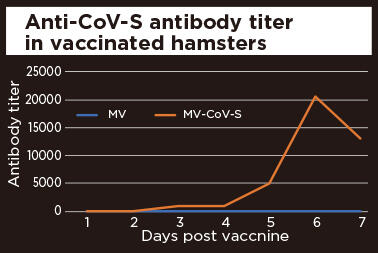
Hamsters were vaccinated twice with measles vaccine (MV) or Covid19-S expressing measles vaccine (MV-CoV-S). Anti-S antibodies were increased highly in the hamsters vaccinated with Covid19-S expressing vaccine. The data of antibody titer was shown as average of 5 hamsters. This result suggested our recombinant vaccine can induce immune response to COVID-19 strongly.
Overcoming shortfalls of live vaccines
Live vaccines are not without drawbacks, however. They use living viruses that can potentially turn toxic once they enter the human body. It therefore takes a longer time to ensure their safety than inactivated vaccines, whose virus particles have no disease-producing capability.
Yoneda's research team has already addressed the safety issue. "The measles virus has been used for measles vaccination around the world since the 1960s," she said. "Since its safety has long been established, there is no problem in using it as a vector." A vector is a means of delivering an antigen that invades human cells to insert the antigen's code. In fact, the Nipah vaccine Yoneda was involved in developing was confirmed safe, and is scheduled to be put to practical use shortly in collaboration with the Coalition of Epidemic Preparedness Innovations (CEPI), a global partnership to accelerate the development of vaccines against emerging infectious diseases.
Another technical issue is how to insert the S protein, the main antigen component in SARS-CoV-2 (the virus triggering COVID-19), into the vector through genetic engineering.
Yoneda used the same technique developed at UTokyo-IIS that was used in developing the Nipah vaccine. "Until recently, it was considered difficult to make genetically engineered viruses from negative-strand RNA viruses like the measles virus," she said. But UTokyo-IIS succeeded several years ago in synthesizing a recombinant virus from measles virus genes with the help of so-called supporting plasmids (genetic structures in a cell that can replicate independently of chromosomes). That made genetic engineering much easier.
Trying to clear last hurdle
Yoneda said the last hurdle - which could be the biggest one - for her team's COVID-19 vaccine is to find a pharmaceutical company that is willing to market the vaccine when all clinical trials are completed. "It costs much more to develop live vaccines because we have to make sure they are safe for people," she said.
Also, the number of vaccine doses required for the global population is much smaller than that for inactivated vaccines, because live vaccines can provide lifelong immunity with a few shots. This sounds like good news, but for many drug makers it means live vaccines are not commercially viable. "We might need a philanthropic organization like the Bill & Melinda Gates Foundation to make this vaccine available to society," Yoneda said, adding that several attempts to persuade drug makers to produce and market her team's COVID-19 vaccine have failed.
She also hopes the Pharmaceuticals and Medical Devices Agency (PMDA), Japan's drug approval authority, will quickly approve her team's vaccine, because the merits of making it widely available outweigh any attendant risks. The United States and many other nations have approved COVID-19 vaccines made by drug makers such as Pfizer/BioNTech and Moderna for emergency use. "We are conducting conversations about our vaccine with PMDA," Yoneda said, adding that her team expects to finish Phase 1 of three-stage clinical testing in a few months after it starts.
Making contributions to society with her research
Yoneda began research on viruses when she was a doctoral student at UTokyo's Graduate School of Agricultural and Life Sciences. Her original interest was not in vaccines, but in how viruses are host-specific, meaning how viruses can cause sickness in certain animals, but not others. She became deeply involved in developing a vaccine after studying the Nipah virus. Yoneda visited an epidemic area in Bangladesh on a separate project to develop a quick test kit for the virus. "I was astonished to see the hygiene and health conditions in the area," she said. "I really wanted to help them with the vaccine we have developed."
Yoneda believes she can also make a difference for people in developing nations with the COVID-19 vaccine she is developing. "It is a huge chance for me to contribute to society," she said. "I want to build on the technologies and knowledge I have acquired so that I can help make the world a better place to live in."
