|
【階層的手法に基づく超分子材料の開発】
Amphiphilic sulfamide as a low-molecular-mass hydrogelator: A novel mode of 3-D networks formed
by hydrogen-bond-directed 2-D sheet assemblies
S. Kabashima, M. Kageyama, T. Okano, I. Yoshikawa, K. Araki, J. Colloid Interface Sci.,408, 107-112 (2013)
 [Abstract] Asymmetrically substituted amphiphilic sulfamide, N-tetradecyl-N′-(6-dimethylaminohexyl)-sulfamide
(3c) having a dimethylamino group at one end of the side chain, showed a strong ability to form
two-dimensional(2-D) sheet-like assemblies by the 2-D hydrogen-bond networks between sulfamide moieties.
Upon protonation of the amino group with acid, the cationic ammonium form of 3c induced effective
hydrogelation (minimum gelation concentration: 0.5 wt%) to yield a translucent, self-standing hydrogel.
Infrared (IR) spectroscopy, X-ray diffraction (XRD), atomic force microscopy (AFM),
scanning electron microscopy (SEM), and transmission electron microscopy (TEM) studies confirmed formation of
the fibrous assemblies of the hydrogen-bond-directed 2-D nanosheets in the hydrogel.
A novel mode of three-dimensional (3-D) networks was formed by branching and recombination of hydrogen-bond networks
and knit-like linkages between the assemblies. The storage and loss moduli of the hydrogel (2 wt%) were measured
to be in the range of 102 and 103 Pa, showing relatively high mechanical stability.
[Abstract] Asymmetrically substituted amphiphilic sulfamide, N-tetradecyl-N′-(6-dimethylaminohexyl)-sulfamide
(3c) having a dimethylamino group at one end of the side chain, showed a strong ability to form
two-dimensional(2-D) sheet-like assemblies by the 2-D hydrogen-bond networks between sulfamide moieties.
Upon protonation of the amino group with acid, the cationic ammonium form of 3c induced effective
hydrogelation (minimum gelation concentration: 0.5 wt%) to yield a translucent, self-standing hydrogel.
Infrared (IR) spectroscopy, X-ray diffraction (XRD), atomic force microscopy (AFM),
scanning electron microscopy (SEM), and transmission electron microscopy (TEM) studies confirmed formation of
the fibrous assemblies of the hydrogen-bond-directed 2-D nanosheets in the hydrogel.
A novel mode of three-dimensional (3-D) networks was formed by branching and recombination of hydrogen-bond networks
and knit-like linkages between the assemblies. The storage and loss moduli of the hydrogel (2 wt%) were measured
to be in the range of 102 and 103 Pa, showing relatively high mechanical stability.
Dry Micromanipulation of Supramolecular Giant Vesicles on a Silicon
Substrate: Highly Stable Hydrogen-Bond-Directed Nanosheet Membrane
H. Sakaino, J. Sawayama, S. Kabashima, I. Yoshikawa and K. Araki, J. Am. Chem. Soc.,134 ,15684-15687 (2012)
 [Abstract] Guanosine derivative 1 forms hydrogen-bond-directed giant vesicles. On a silicon substrate,
the vesicles retain their shape and internal water phase even after removal of external water under vacuum.
Dry manipulation of the micrometer-sized vesicles was carried out via AFM-tip-induced partition and fusion
of the vesicles. For larger vesicles (5-10 μm), external solutions were successfully injected through
a microcapillary inserted into the vesicle in air.
[Abstract] Guanosine derivative 1 forms hydrogen-bond-directed giant vesicles. On a silicon substrate,
the vesicles retain their shape and internal water phase even after removal of external water under vacuum.
Dry manipulation of the micrometer-sized vesicles was carried out via AFM-tip-induced partition and fusion
of the vesicles. For larger vesicles (5-10 μm), external solutions were successfully injected through
a microcapillary inserted into the vesicle in air.
Hydrogen-Bond-Directed Giant Unilamellar Vesicles of Guanosine Derivative: Preparation, Properties, and Fusion
J. Sawayama, H. Sakaino, S. Kabashima, I. Yoshikawa and K. Araki, Langmuir, 27, 8653-8658, (2011)
 [Abstract] By mixing a small volume of THF containing guanosine derivative 1 and tetraethylenegrycol dodecyl
ether (TEGDE) with water and subsequently removing TEGDE by gel permeation chromatography, micrometer-sized giant
unilamellar vesicles (GUV) of 1 were successfully prepared. The vesicle membrane was a 2-D sheet assembly of
thickness 2.5 nm, composed of a 2-D inter-guanine hydrogen-bond network. The GUV dispersion showed high stability
because of a large negative zeta potential, which allowed repeated sedimentation and redispersion by centrifugation
and subsequent gentle agitation. TEGDE-triggered fusion of GUVs took place within 350 ms, which proceeded by fusion
of the vesicle membranes in contact. These unique static and dynamic properties of the GUV membrane assembled by
the 2-D hydrogen-bond network are discussed.
[Abstract] By mixing a small volume of THF containing guanosine derivative 1 and tetraethylenegrycol dodecyl
ether (TEGDE) with water and subsequently removing TEGDE by gel permeation chromatography, micrometer-sized giant
unilamellar vesicles (GUV) of 1 were successfully prepared. The vesicle membrane was a 2-D sheet assembly of
thickness 2.5 nm, composed of a 2-D inter-guanine hydrogen-bond network. The GUV dispersion showed high stability
because of a large negative zeta potential, which allowed repeated sedimentation and redispersion by centrifugation
and subsequent gentle agitation. TEGDE-triggered fusion of GUVs took place within 350 ms, which proceeded by fusion
of the vesicle membranes in contact. These unique static and dynamic properties of the GUV membrane assembled by
the 2-D hydrogen-bond network are discussed.
Hydrogen-Bond-Directed 2-D Sheet Assemblies of Sulfamide Derivatives: Formation of Giant Vesicles with
Patchwork-Like Surface Pattern
S. Kabashima, S. Tanaka, M. Kageyama, I. Yoshikawa and K. Araki, Langmuir, 27, 8950-8955, (2011)
 [Abstract] Sulfamide derivatives showed high ability to form hydrogen-bond-directed two-dimensional (2-D) sheet
assemblies of nanometer thickness. Further, fine-tuning of the side chain structures and preparation conditions
allowed for the formation of micrometer-sized giant vesicles of 4b in water by the simple injection method.
IR and XRD studies indicated that 4b having tetradecyl and oxyethylene-terminated alkyl side chains formed
hydrogen-bond-directed 2-D nanosheet pairs. SEM, AFM, and TEM observation of the dried vesicles revealed
that the vesicle membrane was composed of several lamellar-stacked layers of 2-D nanosheets and showed
a characteristic patchwork-like pattern on the surface.
[Abstract] Sulfamide derivatives showed high ability to form hydrogen-bond-directed two-dimensional (2-D) sheet
assemblies of nanometer thickness. Further, fine-tuning of the side chain structures and preparation conditions
allowed for the formation of micrometer-sized giant vesicles of 4b in water by the simple injection method.
IR and XRD studies indicated that 4b having tetradecyl and oxyethylene-terminated alkyl side chains formed
hydrogen-bond-directed 2-D nanosheet pairs. SEM, AFM, and TEM observation of the dried vesicles revealed
that the vesicle membrane was composed of several lamellar-stacked layers of 2-D nanosheets and showed
a characteristic patchwork-like pattern on the surface.
Novel sulfamide-type low-molecular-mass gelators: gelation of aqueous, organic, and
aqueous/organic biphasic solutions by hydrogen bonddirected 2-D amphiphilic sheet assemblies
N. Maeda, K. Masuda, J. Li, S. Kabashima I. Yoshikawa and K. Araki, Soft Matter, 6, 5305-5307, (2010)
 [Abstract] A novel asymmetrically-substituted sulfamide forms hydrogen bond-directed amphiphilic 2-D sheet assemblies
and shows high ability to induce gelation of variety of solvents including polar water, nonpolar dodecane, and
aqueous/organic biphasic systems.
[Abstract] A novel asymmetrically-substituted sulfamide forms hydrogen bond-directed amphiphilic 2-D sheet assemblies
and shows high ability to induce gelation of variety of solvents including polar water, nonpolar dodecane, and
aqueous/organic biphasic systems.
Hydrogen-Bond-Directed Giant Vesicles of Guanosine Derivatives in Water: Formation, Structure, and Stability
J. Sawayama, I. Yoshikawa and K. Araki, Langmuir, 26, 8030-8035, (2010)
 [Abstract] Hydrogen-bond-directed giant supramolecular vesicles of an alkylsilylated deoxyguanosine derivative,
2a, were prepared faciley by mixing a small volume of a 2a/THF solution with water.
The formation of 2-D inter-guanine hydrogen-bond networks of 2a within the vesicles
was indicated by IR spectra. The vesicle solution was stable enough for more than 30 days,
in a wide range of temperatures, and between pH 4 and 10 without showing lysis, fusion,
precipitation, or leakage of the encapsulated fluorescent probe. In a typical micrometer-sized
vesicle, a sufficiently large internal water phase for encapsulating water-soluble substances
was surrounded by a multilamellar membrane 15-20 nm in thickness, which was composed of
6-9 layers of 2-D hydrogen-bond-directed sheet assemblies. AC-mode AFM observation of the vesicle
on a silicon substrate further demonstrated the high stability and deformable properties of
the vesicle membrane under vacuum or mechanical stress. The formation and properties of the
vesicle membrane in water were analyzed from the viewpoint of the 2-D hydrogen-bond-directed
sheet assemblies, and the scope of the design principle to use nonpolar soft segments as
the shielding units of the hydrogen-bond networks in water is discussed.
[Abstract] Hydrogen-bond-directed giant supramolecular vesicles of an alkylsilylated deoxyguanosine derivative,
2a, were prepared faciley by mixing a small volume of a 2a/THF solution with water.
The formation of 2-D inter-guanine hydrogen-bond networks of 2a within the vesicles
was indicated by IR spectra. The vesicle solution was stable enough for more than 30 days,
in a wide range of temperatures, and between pH 4 and 10 without showing lysis, fusion,
precipitation, or leakage of the encapsulated fluorescent probe. In a typical micrometer-sized
vesicle, a sufficiently large internal water phase for encapsulating water-soluble substances
was surrounded by a multilamellar membrane 15-20 nm in thickness, which was composed of
6-9 layers of 2-D hydrogen-bond-directed sheet assemblies. AC-mode AFM observation of the vesicle
on a silicon substrate further demonstrated the high stability and deformable properties of
the vesicle membrane under vacuum or mechanical stress. The formation and properties of the
vesicle membrane in water were analyzed from the viewpoint of the 2-D hydrogen-bond-directed
sheet assemblies, and the scope of the design principle to use nonpolar soft segments as
the shielding units of the hydrogen-bond networks in water is discussed.
 Highly Stable Giant Supramolecular Vesicles Composed of 2D Hydrogen-Bonded Sheet Structures of Guanosine Derivatives
Highly Stable Giant Supramolecular Vesicles Composed of 2D Hydrogen-Bonded Sheet Structures of Guanosine Derivatives
I. Yoshikawa, J. Sawayama, K. Araki, Angew. Chem. Int. Ed., 47, 1038-1041 (2008)
Molecular design to protect a 2-D hydrogen-bond network with non-polar shielding layers
allowed the fabrication of micrometer-scale supramolecular vesicles in water. These
hydrogen bond-directed giant vesicles and their dispersions in aqueous media showed
sufficiently high stability under various conditions.
Nucleoside-based organogelators: gelation by the G-G base pair formation of alkylsilylated guanosine derivatives
I.Yoshikawa, S. Yanagi, Y. Yamaji, K. Araki, Tetrahedron, 63, 7474-7481 (2007)
 [Abstract] 2',3'-O-Isopropylideneguanosine derivatives having a bulky alkyl-silyl moiety
showed excellent gelation ability in alkane solvents. IR spectra of the gel clearly
showed the absence of hydrogen bonding interaction at the C(6)O position and,
together with a CD study, a G-G base pair formation by double N(2)H--N(3) and additional
N(2)H--O(2') hydrogen bonds was indicated. X-ray diffraction and SEM studies of the xerogel
and AFM observation of the transferred gel suggested the formation of a two-dimensional
supramolecular assembly 2 nm in thickness. The G-G base pair formation is discussed in terms
of the molecular packing in the two-dimensional assemblies.
[Abstract] 2',3'-O-Isopropylideneguanosine derivatives having a bulky alkyl-silyl moiety
showed excellent gelation ability in alkane solvents. IR spectra of the gel clearly
showed the absence of hydrogen bonding interaction at the C(6)O position and,
together with a CD study, a G-G base pair formation by double N(2)H--N(3) and additional
N(2)H--O(2') hydrogen bonds was indicated. X-ray diffraction and SEM studies of the xerogel
and AFM observation of the transferred gel suggested the formation of a two-dimensional
supramolecular assembly 2 nm in thickness. The G-G base pair formation is discussed in terms
of the molecular packing in the two-dimensional assemblies.
TOPへ戻る
【新規蛍光性化合物群の開発と機能評価】
Colorless, Transparent, Dye-Doped Polymer Films Exhibiting Tunable Luminescence Color: Controlling
the Dual-Color Luminescence of 2-(2'-Hydroxyphenyl)imidazo[1,2-a]pyridine Derivatives with the Surrounding Matrix
S. Furukawa, H. Shono, T. Mutai, K. Araki, ACS Appl. Mater. Interfaces, 6, 16065-16070 (2014)
 [Abstract] Colorless, transparent, polymer films including 2-(2'-hydroxyphenyl)imidazo[1,2-a]pyridine (HPIP) derivatives,
1 and 2, are prepared by a spin-coating method. The observed emission spectra upon photoexcitation of
these polymer films were composed of dual emission bands: the normal luminescence at 370–410 nm (purple) and
the greatly Stokes-shifted emission at 520–580 nm (yellow) assigned as the excited-state intramolecular proton
transfer (ESIPT) luminescence. Relative intensity of the two emissions varied according to the polymer matrices,
resulting in change in the luminescent color of the dye-doped polymer films. Particularly, the luminescence properties of
6-cyano HPIP, 2, are highly susceptible to the surrounding environment, and therefore successfully tuned
to produce a wide range of colors, from purple to orange, by changing its concentration within and the type of
the polymer matrix. This observation can be ascribed to the formation of a relatively weak intramolecular
hydrogen bond resulting from the electron-withdrawing 6-cyano group. Thus, we demonstrate large variations
in emission color can be achieved using interactions of the single component with the surrounding matrix.
These results offer promise as a convenient and effective method for a wide-range tuning the luminescence
colors of dye-doped polymer films.
[Abstract] Colorless, transparent, polymer films including 2-(2'-hydroxyphenyl)imidazo[1,2-a]pyridine (HPIP) derivatives,
1 and 2, are prepared by a spin-coating method. The observed emission spectra upon photoexcitation of
these polymer films were composed of dual emission bands: the normal luminescence at 370–410 nm (purple) and
the greatly Stokes-shifted emission at 520–580 nm (yellow) assigned as the excited-state intramolecular proton
transfer (ESIPT) luminescence. Relative intensity of the two emissions varied according to the polymer matrices,
resulting in change in the luminescent color of the dye-doped polymer films. Particularly, the luminescence properties of
6-cyano HPIP, 2, are highly susceptible to the surrounding environment, and therefore successfully tuned
to produce a wide range of colors, from purple to orange, by changing its concentration within and the type of
the polymer matrix. This observation can be ascribed to the formation of a relatively weak intramolecular
hydrogen bond resulting from the electron-withdrawing 6-cyano group. Thus, we demonstrate large variations
in emission color can be achieved using interactions of the single component with the surrounding matrix.
These results offer promise as a convenient and effective method for a wide-range tuning the luminescence
colors of dye-doped polymer films.
Three-color polymorph-dependent luminescence: crystallographic analysis and theoretical study on excited-state
intramolecular proton transfer (ESIPT) luminescence of cyano-substituted imidazo[1,2-a]pyridine
T. Mutai, H. Shono, Y. Shigemitsu, K. Araki, CrystEngComm, 16, 3890-3895 (2014)
 [Abstract] Yellow, orange, and red luminescence can be achieved by controlling the crystalline polymorphs of 6-cyano HPIP (2).
[Abstract] Yellow, orange, and red luminescence can be achieved by controlling the crystalline polymorphs of 6-cyano HPIP (2).
Sterically induced polymorphism: ON-OFF control of excited-state intramolecular proton transfer (ESIPT)
luminescence of 1-methyl-2-(2'-hydroxyphenyl)benzimidazole
T. Shida, T. Mutai, K. Araki, CrystEngComm, 15, 10179-10182 (2013)
 [Abstract] Introduction of ‘appropriate’ steric hindrance into 2-(2′-hydroxyphenyl)benzimidazole leads
to polymorphic crystals that exhibit strong (Φ = 0.74) and practically no (Φ = 0.007) ESIPT luminescence.
[Abstract] Introduction of ‘appropriate’ steric hindrance into 2-(2′-hydroxyphenyl)benzimidazole leads
to polymorphic crystals that exhibit strong (Φ = 0.74) and practically no (Φ = 0.007) ESIPT luminescence.
Tuning of Excited-State Intramolecular Proton Transfer (ESIPT) Fluorescence of Imidazo[1,2-a]pyridine
in Rigid Matrices by Substitution Effect
T. Mutai, H. Sawatani, T. Shida, H. Shono, K. Araki, J. Org. Chem., 78, 2482-2489 (2013)
 [Abstract] 2-(2'-Hydroxyphenyl)imidazo[1,2-a]pyridine (HPIP, 1) and its derivatives are synthesized, and
their fluorescence properties are studied. Although all the compounds show faint dual emission
(Φ ≈ 0.01), which is assigned to the normal and excited-state intramolecular proton transfer (ESIPT)
fluorescence in a fluid solution, they generally display efficient ESIPT fluorescence (Φ up to 0.6)
in a polymer matrix. The introduction of electron-donating and electron-withdrawing groups into the phenyl
ring causes blue and red shifts of the ESIPT fluorescence emission band, respectively. On the other hand,
the introduction of such groups into the imidazopyridine part results in fluorescence shifts in the opposite
directions. The results of ab initio quantum chemical calculations of the intramolecular proton-transferred (IPT)
state are well in line with the ESIPT fluorescence energies. The plots of the calculated highest occupied molecular
orbital (HOMO) and lowest unoccupied molecular orbital (LUMO) energy levels against the Hammett substituent constants
(σ) show good linearity with different slopes, which can rationalize the effect of the substituent and its position
on the IPT state. Therefore, we have developed a series of HPIPs as new ESIPT fluorescent compounds and demonstrate
that ESIPT fluorescence properties would be rationally tuned using quantum chemical methods.
[Abstract] 2-(2'-Hydroxyphenyl)imidazo[1,2-a]pyridine (HPIP, 1) and its derivatives are synthesized, and
their fluorescence properties are studied. Although all the compounds show faint dual emission
(Φ ≈ 0.01), which is assigned to the normal and excited-state intramolecular proton transfer (ESIPT)
fluorescence in a fluid solution, they generally display efficient ESIPT fluorescence (Φ up to 0.6)
in a polymer matrix. The introduction of electron-donating and electron-withdrawing groups into the phenyl
ring causes blue and red shifts of the ESIPT fluorescence emission band, respectively. On the other hand,
the introduction of such groups into the imidazopyridine part results in fluorescence shifts in the opposite
directions. The results of ab initio quantum chemical calculations of the intramolecular proton-transferred (IPT)
state are well in line with the ESIPT fluorescence energies. The plots of the calculated highest occupied molecular
orbital (HOMO) and lowest unoccupied molecular orbital (LUMO) energy levels against the Hammett substituent constants
(σ) show good linearity with different slopes, which can rationalize the effect of the substituent and its position
on the IPT state. Therefore, we have developed a series of HPIPs as new ESIPT fluorescent compounds and demonstrate
that ESIPT fluorescence properties would be rationally tuned using quantum chemical methods.
Propylamino-connected Fluorescent Terpyridine Dimer and Trimer: Syntheses, Photophysical Properties
and Formation of Duplex-Type Complexes with Cd(II)
Y. Kamoya, K. Kojima, G. Tanaka, R. Tanaka, T. Mutai and K. Araki,
Org. Biomol. Chem., 10, 8895-8902 (2012)
 [Abstract] By using propylamine as a connector, a dimer (L2) and trimer (L3) of 2,2':6',2''-terpyridine (tpy) are
synthesized. They showed the lowest energy π-π* absorption at 354 nm and blue fluorescence at 420 nm
with a moderate quantum yield in solution. Spectral titrations, electrospray ionization mass spectrometry
(ESI-MS), 1 H-1H rotating-frame nuclear Overhauser effect correlation spectroscopy (ROESY), and
structure simulation (MOPAC/PM5) indicated that both L2 and L3 form duplex-type multi-component
complexes with Cd(II), [Cd2(L2)2]4+ and [Cd3(L3)2]6+,
respectively. While the luminescence of these was quite weak in solution (quantum yield Φ < 0.005), the complexes
showed enhanced emission in the solid state with remarkable increase of the quantum yield (Φ = 0.13).
[Abstract] By using propylamine as a connector, a dimer (L2) and trimer (L3) of 2,2':6',2''-terpyridine (tpy) are
synthesized. They showed the lowest energy π-π* absorption at 354 nm and blue fluorescence at 420 nm
with a moderate quantum yield in solution. Spectral titrations, electrospray ionization mass spectrometry
(ESI-MS), 1 H-1H rotating-frame nuclear Overhauser effect correlation spectroscopy (ROESY), and
structure simulation (MOPAC/PM5) indicated that both L2 and L3 form duplex-type multi-component
complexes with Cd(II), [Cd2(L2)2]4+ and [Cd3(L3)2]6+,
respectively. While the luminescence of these was quite weak in solution (quantum yield Φ < 0.005), the complexes
showed enhanced emission in the solid state with remarkable increase of the quantum yield (Φ = 0.13).
Fabrication of Colorless Organic Materials Exhibiting White Luminescence Using Normal and
Excited-State Intramolecular Proton Transfer Processes
H. Shono, T. Ohkawa, H. Tomoda, T. Mutai and K. Araki, ACS Appl. Mater. Interfaces, 3, 654-657 (2011)
 [Abstract] Organic, white luminescent materials were fabricated using a mixture of proton-transfer and
nonproton-transfer fluorophores. 2'-Methoxy and 2'-hydroxy derivatives of 2-phenylimidazo[1,2-a]pyridine
(PIP) have similar UV-absorption properties; however, they exhibit mechanistically different luminescence
respectively ascribable to the normal (~420 nm) and excited-state intramolecular proton transfer processes
(~530 nm) in the solid state. UV-irradiation of mixed solids excites both components concurrently and results
in efficient white luminescence composed of two independent emissions without involving energy transfer process.
White luminescent solids are easily transformed into vapor-deposited films under mild conditions, and
a colorless and transparent thin film by dissolving in PMMA.
[Abstract] Organic, white luminescent materials were fabricated using a mixture of proton-transfer and
nonproton-transfer fluorophores. 2'-Methoxy and 2'-hydroxy derivatives of 2-phenylimidazo[1,2-a]pyridine
(PIP) have similar UV-absorption properties; however, they exhibit mechanistically different luminescence
respectively ascribable to the normal (~420 nm) and excited-state intramolecular proton transfer processes
(~530 nm) in the solid state. UV-irradiation of mixed solids excites both components concurrently and results
in efficient white luminescence composed of two independent emissions without involving energy transfer process.
White luminescent solids are easily transformed into vapor-deposited films under mild conditions, and
a colorless and transparent thin film by dissolving in PMMA.
 Tuning of fluorescence properties of aminoterpyridine fluorophores by N-substitution
Tuning of fluorescence properties of aminoterpyridine fluorophores by N-substitution
J.-D. Cheon, T. Mutai, K. Araki, Org. Biomol. Chem., 5, 2762-2766 (2007)
 [Abstract] Several N-alkyl and N-phenyl derivatives of 6-amino- and 6,6-diamino-2,2:6,2-terpyridine
were synthesized, and their fluorescence properties were studied. A successive red-shift
was observed as the number of the N-substituted groups increased. It was also shown that
the susceptivity of the fluorophores to a solvent varied considerably according to the mode
of the N-substitution. While the monoamino-tpys (tpy: 2,2:6,2-terpyridine) suffered almost
complete quenching of their fluorescence in ethanol, the fully N-alkylated diamino-tpys
retained their fluorescence. The results show that N-substitution is a useful way to tune
both the radiation energy and solvent susceptivity of the fluorescence of the amino-tpys.
[Abstract] Several N-alkyl and N-phenyl derivatives of 6-amino- and 6,6-diamino-2,2:6,2-terpyridine
were synthesized, and their fluorescence properties were studied. A successive red-shift
was observed as the number of the N-substituted groups increased. It was also shown that
the susceptivity of the fluorophores to a solvent varied considerably according to the mode
of the N-substitution. While the monoamino-tpys (tpy: 2,2:6,2-terpyridine) suffered almost
complete quenching of their fluorescence in ethanol, the fully N-alkylated diamino-tpys
retained their fluorescence. The results show that N-substitution is a useful way to tune
both the radiation energy and solvent susceptivity of the fluorescence of the amino-tpys.
Fluorescent oligopyridines and their photo-functionality as tunable fluorophores
T. Mutai, K. Araki, Current Org. Chem. 11, 195-211 (2007)
TOPへ戻る
【分子集積構造変化を利用した有機個体発光制御】
Solid-state luminescence of tetraphenylpyrene derivatives: mechano/vapochromic luminescence of 1,3,6,8-tetra(4'-carboxyphenyl)pyrene
S. Yamaguchi, I. Yoshikawa, T. Mutai and K. Araki, J. Mater. Chem., 22, 20065-20070 (2012)
 [Abstract] The effect of mechanical stress on the solid-state luminescence of 3, a carboxylic acid derivative of
1,3,6,8-tetraphenylpyrene (TPPy), is studied. Grinding the powdery solid of 3 in a mortar results in a
blue shift of luminescence (from yellow to green) instead of the red shift observed for amide (1) and
ester (2) derivatives of TPPy. The initial yellow luminescence is recovered by exposure to solvent
vapour. In contrast, application of compressive stress using an oil press causes practically no change of
the initial yellow luminescence of 3. The yellow luminescence is ascribed to the dimeric form of 3, while
the green luminescence is ascribed to the monomeric state. The macroscale shear stress applied by
grinding induces the dimer-to-monomer transition at the molecular level, which causes the blue shift of
the luminescence. Based on the results, the mechanisms of macroscale compressive and shear stresses to
induce alteration of nanoscale molecular packing and luminescent properties are discussed.
[Abstract] The effect of mechanical stress on the solid-state luminescence of 3, a carboxylic acid derivative of
1,3,6,8-tetraphenylpyrene (TPPy), is studied. Grinding the powdery solid of 3 in a mortar results in a
blue shift of luminescence (from yellow to green) instead of the red shift observed for amide (1) and
ester (2) derivatives of TPPy. The initial yellow luminescence is recovered by exposure to solvent
vapour. In contrast, application of compressive stress using an oil press causes practically no change of
the initial yellow luminescence of 3. The yellow luminescence is ascribed to the dimeric form of 3, while
the green luminescence is ascribed to the monomeric state. The macroscale shear stress applied by
grinding induces the dimer-to-monomer transition at the molecular level, which causes the blue shift of
the luminescence. Based on the results, the mechanisms of macroscale compressive and shear stresses to
induce alteration of nanoscale molecular packing and luminescent properties are discussed.
Piezochromic luminescence of amide and ester derivatives of tetraphenylpyrene -role of amide hydrogen bonds
in sensitive piezochromic response
M. Sase, S. Yamaguchi, Y. Sagara, I. Yoshikawa, T. Mutai and K. Araki, J. Mater Chem., 21, 8347-8354 (2011)
 [Abstract] A series of amide and ester derivatives of 1,3,6,8-tetraphenylpyrene (TPPy) were prepared, and
their solid-state luminescence, molecular packing, and piezochromic responses were studied in order to improve
and refine the design concept of piezochromic luminescent materials based on the use of multiple hydrogen bonds
and close packing of disk-shaped aromatic cores as competing factors in the control of molecular packing.
The amide derivative of TPPy having hexyl side chains showed a sensitive luminescence response at a
relatively low applied pressure, which was ascribed to the hydrogen bond-directed H-type columnar packing.
Application of higher pressure induced considerable destruction of the hydrogen bond-directed structure,
leading to the state where close packing was the overriding factor directing the molecular assembly and
showed similar piezochromic response to that of the ester derivative. Based on the results, the role of
the amide hydrogen bonds and the side chains was discussed in detail.
[Abstract] A series of amide and ester derivatives of 1,3,6,8-tetraphenylpyrene (TPPy) were prepared, and
their solid-state luminescence, molecular packing, and piezochromic responses were studied in order to improve
and refine the design concept of piezochromic luminescent materials based on the use of multiple hydrogen bonds
and close packing of disk-shaped aromatic cores as competing factors in the control of molecular packing.
The amide derivative of TPPy having hexyl side chains showed a sensitive luminescence response at a
relatively low applied pressure, which was ascribed to the hydrogen bond-directed H-type columnar packing.
Application of higher pressure induced considerable destruction of the hydrogen bond-directed structure,
leading to the state where close packing was the overriding factor directing the molecular assembly and
showed similar piezochromic response to that of the ester derivative. Based on the results, the role of
the amide hydrogen bonds and the side chains was discussed in detail.
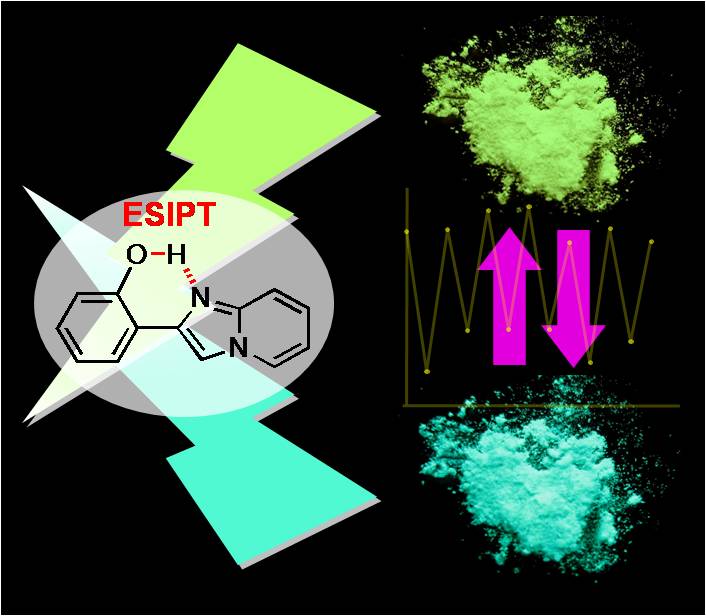 Switching of polymorph-dependent ESIPT luminescence of an imidazo[1,2-a]pyridine derivative
Switching of polymorph-dependent ESIPT luminescence of an imidazo[1,2-a]pyridine derivative
T. Mutai, H. Tomoda, T. Ohkawa, Y. Yabe, K. Araki, Angew. Chem. Int. Ed. 47, 9522-9524 (2008)
[Abstract] Reproducible switching of the polymorph-dependent excited-state intramol. proton-transfer (ESIPT)
luminescence of an imidazo[1,2-a]pyridine between blue-green and yellow is achieved by thermal control of its
solid-state mol. packing. Thus, ESIPT is a promising mechanism for packing-to-luminescence transduction and
amplification that offers a novel design concept for tunable org. luminescent solids.
Material Design for Piezochromic Luminescence: Hydrogen-Bond-Directed Assemblies of a Pyrene Derivative
Y. Sagara, T. Mutai, I. Yoshikawa, K. Araki, J. Am. Chem. Soc. 129, 1520-1521 (2007)
 [Abstract] A novel design principle for materials showing piezochromic luminescence is proposed. Amide-substituted
tetraphenylpyrene C6TPPy, composed of a planar fluorescent core and multiple hydrogen-binding sites,
displays a remarkable change and restoration of its luminescence color by pressing with a spatula and
heating, respectively.
[Abstract] A novel design principle for materials showing piezochromic luminescence is proposed. Amide-substituted
tetraphenylpyrene C6TPPy, composed of a planar fluorescent core and multiple hydrogen-binding sites,
displays a remarkable change and restoration of its luminescence color by pressing with a spatula and
heating, respectively.
 Reproducible on-off switching of solid-state luminescence by controlling molecular packing through
heat-mode interconversion
Reproducible on-off switching of solid-state luminescence by controlling molecular packing through
heat-mode interconversion
T. Mutai, H. Satou, K. Araki, Nature Mater. 4, 685-687 (2005)
Switching of Solid-state Organic Luminescence by Interconversion of Mode of Molecular Assemblies
There are increasing demands for new solid-state organic luminescent materials. Luminescence
of solid terpyridine can be controlled by the mode of molecular assembly without modification of
the chemical structure. It forms either a highly luminescent or non-luminescent crystal, and
these two crystals are interconverted to each other by an external heating stimulus.
The luminescence is concomitantly switched on and off with high reliability,
showing possible application for heat-mode recording.
TOPへ戻る
【機能性金属錯体の開発と単分子デバイスへの応用】
Molecular switches for electron and energy transfer processes based on metal complexes
J. Otsuki, T. Akasaka, K. Araki, Coordination Chem. Rev. 252, 32-56 (2008)
Abstract : Molecular switches based on metal complexes, in which electron and/or energy transfer processes are switched
on and off or significantly modulated by externally applied stimuli, such as electrons (redox reactions),
light, and chemical substances (protons, ions, and molecules), are reviewed. Also described are single-molecule
transistors, in which conductance through a single metal complex is modulated by applied gate voltages.
TOPへ戻る
|
 [Abstract] Asymmetrically substituted amphiphilic sulfamide, N-tetradecyl-N′-(6-dimethylaminohexyl)-sulfamide
(3c) having a dimethylamino group at one end of the side chain, showed a strong ability to form
two-dimensional(2-D) sheet-like assemblies by the 2-D hydrogen-bond networks between sulfamide moieties.
Upon protonation of the amino group with acid, the cationic ammonium form of 3c induced effective
hydrogelation (minimum gelation concentration: 0.5 wt%) to yield a translucent, self-standing hydrogel.
Infrared (IR) spectroscopy, X-ray diffraction (XRD), atomic force microscopy (AFM),
scanning electron microscopy (SEM), and transmission electron microscopy (TEM) studies confirmed formation of
the fibrous assemblies of the hydrogen-bond-directed 2-D nanosheets in the hydrogel.
A novel mode of three-dimensional (3-D) networks was formed by branching and recombination of hydrogen-bond networks
and knit-like linkages between the assemblies. The storage and loss moduli of the hydrogel (2 wt%) were measured
to be in the range of 102 and 103 Pa, showing relatively high mechanical stability.
[Abstract] Asymmetrically substituted amphiphilic sulfamide, N-tetradecyl-N′-(6-dimethylaminohexyl)-sulfamide
(3c) having a dimethylamino group at one end of the side chain, showed a strong ability to form
two-dimensional(2-D) sheet-like assemblies by the 2-D hydrogen-bond networks between sulfamide moieties.
Upon protonation of the amino group with acid, the cationic ammonium form of 3c induced effective
hydrogelation (minimum gelation concentration: 0.5 wt%) to yield a translucent, self-standing hydrogel.
Infrared (IR) spectroscopy, X-ray diffraction (XRD), atomic force microscopy (AFM),
scanning electron microscopy (SEM), and transmission electron microscopy (TEM) studies confirmed formation of
the fibrous assemblies of the hydrogen-bond-directed 2-D nanosheets in the hydrogel.
A novel mode of three-dimensional (3-D) networks was formed by branching and recombination of hydrogen-bond networks
and knit-like linkages between the assemblies. The storage and loss moduli of the hydrogel (2 wt%) were measured
to be in the range of 102 and 103 Pa, showing relatively high mechanical stability.
 [Abstract] Guanosine derivative 1 forms hydrogen-bond-directed giant vesicles. On a silicon substrate,
the vesicles retain their shape and internal water phase even after removal of external water under vacuum.
Dry manipulation of the micrometer-sized vesicles was carried out via AFM-tip-induced partition and fusion
of the vesicles. For larger vesicles (5-10 μm), external solutions were successfully injected through
a microcapillary inserted into the vesicle in air.
[Abstract] Guanosine derivative 1 forms hydrogen-bond-directed giant vesicles. On a silicon substrate,
the vesicles retain their shape and internal water phase even after removal of external water under vacuum.
Dry manipulation of the micrometer-sized vesicles was carried out via AFM-tip-induced partition and fusion
of the vesicles. For larger vesicles (5-10 μm), external solutions were successfully injected through
a microcapillary inserted into the vesicle in air.
 [Abstract] By mixing a small volume of THF containing guanosine derivative 1 and tetraethylenegrycol dodecyl
ether (TEGDE) with water and subsequently removing TEGDE by gel permeation chromatography, micrometer-sized giant
unilamellar vesicles (GUV) of 1 were successfully prepared. The vesicle membrane was a 2-D sheet assembly of
thickness 2.5 nm, composed of a 2-D inter-guanine hydrogen-bond network. The GUV dispersion showed high stability
because of a large negative zeta potential, which allowed repeated sedimentation and redispersion by centrifugation
and subsequent gentle agitation. TEGDE-triggered fusion of GUVs took place within 350 ms, which proceeded by fusion
of the vesicle membranes in contact. These unique static and dynamic properties of the GUV membrane assembled by
the 2-D hydrogen-bond network are discussed.
[Abstract] By mixing a small volume of THF containing guanosine derivative 1 and tetraethylenegrycol dodecyl
ether (TEGDE) with water and subsequently removing TEGDE by gel permeation chromatography, micrometer-sized giant
unilamellar vesicles (GUV) of 1 were successfully prepared. The vesicle membrane was a 2-D sheet assembly of
thickness 2.5 nm, composed of a 2-D inter-guanine hydrogen-bond network. The GUV dispersion showed high stability
because of a large negative zeta potential, which allowed repeated sedimentation and redispersion by centrifugation
and subsequent gentle agitation. TEGDE-triggered fusion of GUVs took place within 350 ms, which proceeded by fusion
of the vesicle membranes in contact. These unique static and dynamic properties of the GUV membrane assembled by
the 2-D hydrogen-bond network are discussed.
 [Abstract] Sulfamide derivatives showed high ability to form hydrogen-bond-directed two-dimensional (2-D) sheet
assemblies of nanometer thickness. Further, fine-tuning of the side chain structures and preparation conditions
allowed for the formation of micrometer-sized giant vesicles of 4b in water by the simple injection method.
IR and XRD studies indicated that 4b having tetradecyl and oxyethylene-terminated alkyl side chains formed
hydrogen-bond-directed 2-D nanosheet pairs. SEM, AFM, and TEM observation of the dried vesicles revealed
that the vesicle membrane was composed of several lamellar-stacked layers of 2-D nanosheets and showed
a characteristic patchwork-like pattern on the surface.
[Abstract] Sulfamide derivatives showed high ability to form hydrogen-bond-directed two-dimensional (2-D) sheet
assemblies of nanometer thickness. Further, fine-tuning of the side chain structures and preparation conditions
allowed for the formation of micrometer-sized giant vesicles of 4b in water by the simple injection method.
IR and XRD studies indicated that 4b having tetradecyl and oxyethylene-terminated alkyl side chains formed
hydrogen-bond-directed 2-D nanosheet pairs. SEM, AFM, and TEM observation of the dried vesicles revealed
that the vesicle membrane was composed of several lamellar-stacked layers of 2-D nanosheets and showed
a characteristic patchwork-like pattern on the surface.
 [Abstract] A novel asymmetrically-substituted sulfamide forms hydrogen bond-directed amphiphilic 2-D sheet assemblies
and shows high ability to induce gelation of variety of solvents including polar water, nonpolar dodecane, and
aqueous/organic biphasic systems.
[Abstract] A novel asymmetrically-substituted sulfamide forms hydrogen bond-directed amphiphilic 2-D sheet assemblies
and shows high ability to induce gelation of variety of solvents including polar water, nonpolar dodecane, and
aqueous/organic biphasic systems.
 [Abstract] Hydrogen-bond-directed giant supramolecular vesicles of an alkylsilylated deoxyguanosine derivative,
2a, were prepared faciley by mixing a small volume of a 2a/THF solution with water.
The formation of 2-D inter-guanine hydrogen-bond networks of 2a within the vesicles
was indicated by IR spectra. The vesicle solution was stable enough for more than 30 days,
in a wide range of temperatures, and between pH 4 and 10 without showing lysis, fusion,
precipitation, or leakage of the encapsulated fluorescent probe. In a typical micrometer-sized
vesicle, a sufficiently large internal water phase for encapsulating water-soluble substances
was surrounded by a multilamellar membrane 15-20 nm in thickness, which was composed of
6-9 layers of 2-D hydrogen-bond-directed sheet assemblies. AC-mode AFM observation of the vesicle
on a silicon substrate further demonstrated the high stability and deformable properties of
the vesicle membrane under vacuum or mechanical stress. The formation and properties of the
vesicle membrane in water were analyzed from the viewpoint of the 2-D hydrogen-bond-directed
sheet assemblies, and the scope of the design principle to use nonpolar soft segments as
the shielding units of the hydrogen-bond networks in water is discussed.
[Abstract] Hydrogen-bond-directed giant supramolecular vesicles of an alkylsilylated deoxyguanosine derivative,
2a, were prepared faciley by mixing a small volume of a 2a/THF solution with water.
The formation of 2-D inter-guanine hydrogen-bond networks of 2a within the vesicles
was indicated by IR spectra. The vesicle solution was stable enough for more than 30 days,
in a wide range of temperatures, and between pH 4 and 10 without showing lysis, fusion,
precipitation, or leakage of the encapsulated fluorescent probe. In a typical micrometer-sized
vesicle, a sufficiently large internal water phase for encapsulating water-soluble substances
was surrounded by a multilamellar membrane 15-20 nm in thickness, which was composed of
6-9 layers of 2-D hydrogen-bond-directed sheet assemblies. AC-mode AFM observation of the vesicle
on a silicon substrate further demonstrated the high stability and deformable properties of
the vesicle membrane under vacuum or mechanical stress. The formation and properties of the
vesicle membrane in water were analyzed from the viewpoint of the 2-D hydrogen-bond-directed
sheet assemblies, and the scope of the design principle to use nonpolar soft segments as
the shielding units of the hydrogen-bond networks in water is discussed.
 Highly Stable Giant Supramolecular Vesicles Composed of 2D Hydrogen-Bonded Sheet Structures of Guanosine Derivatives
Highly Stable Giant Supramolecular Vesicles Composed of 2D Hydrogen-Bonded Sheet Structures of Guanosine Derivatives [Abstract] 2',3'-O-Isopropylideneguanosine derivatives having a bulky alkyl-silyl moiety
showed excellent gelation ability in alkane solvents. IR spectra of the gel clearly
showed the absence of hydrogen bonding interaction at the C(6)O position and,
together with a CD study, a G-G base pair formation by double N(2)H--N(3) and additional
N(2)H--O(2') hydrogen bonds was indicated. X-ray diffraction and SEM studies of the xerogel
and AFM observation of the transferred gel suggested the formation of a two-dimensional
supramolecular assembly 2 nm in thickness. The G-G base pair formation is discussed in terms
of the molecular packing in the two-dimensional assemblies.
[Abstract] 2',3'-O-Isopropylideneguanosine derivatives having a bulky alkyl-silyl moiety
showed excellent gelation ability in alkane solvents. IR spectra of the gel clearly
showed the absence of hydrogen bonding interaction at the C(6)O position and,
together with a CD study, a G-G base pair formation by double N(2)H--N(3) and additional
N(2)H--O(2') hydrogen bonds was indicated. X-ray diffraction and SEM studies of the xerogel
and AFM observation of the transferred gel suggested the formation of a two-dimensional
supramolecular assembly 2 nm in thickness. The G-G base pair formation is discussed in terms
of the molecular packing in the two-dimensional assemblies. [Abstract] Colorless, transparent, polymer films including 2-(2'-hydroxyphenyl)imidazo[1,2-a]pyridine (HPIP) derivatives,
1 and 2, are prepared by a spin-coating method. The observed emission spectra upon photoexcitation of
these polymer films were composed of dual emission bands: the normal luminescence at 370–410 nm (purple) and
the greatly Stokes-shifted emission at 520–580 nm (yellow) assigned as the excited-state intramolecular proton
transfer (ESIPT) luminescence. Relative intensity of the two emissions varied according to the polymer matrices,
resulting in change in the luminescent color of the dye-doped polymer films. Particularly, the luminescence properties of
6-cyano HPIP, 2, are highly susceptible to the surrounding environment, and therefore successfully tuned
to produce a wide range of colors, from purple to orange, by changing its concentration within and the type of
the polymer matrix. This observation can be ascribed to the formation of a relatively weak intramolecular
hydrogen bond resulting from the electron-withdrawing 6-cyano group. Thus, we demonstrate large variations
in emission color can be achieved using interactions of the single component with the surrounding matrix.
These results offer promise as a convenient and effective method for a wide-range tuning the luminescence
colors of dye-doped polymer films.
[Abstract] Colorless, transparent, polymer films including 2-(2'-hydroxyphenyl)imidazo[1,2-a]pyridine (HPIP) derivatives,
1 and 2, are prepared by a spin-coating method. The observed emission spectra upon photoexcitation of
these polymer films were composed of dual emission bands: the normal luminescence at 370–410 nm (purple) and
the greatly Stokes-shifted emission at 520–580 nm (yellow) assigned as the excited-state intramolecular proton
transfer (ESIPT) luminescence. Relative intensity of the two emissions varied according to the polymer matrices,
resulting in change in the luminescent color of the dye-doped polymer films. Particularly, the luminescence properties of
6-cyano HPIP, 2, are highly susceptible to the surrounding environment, and therefore successfully tuned
to produce a wide range of colors, from purple to orange, by changing its concentration within and the type of
the polymer matrix. This observation can be ascribed to the formation of a relatively weak intramolecular
hydrogen bond resulting from the electron-withdrawing 6-cyano group. Thus, we demonstrate large variations
in emission color can be achieved using interactions of the single component with the surrounding matrix.
These results offer promise as a convenient and effective method for a wide-range tuning the luminescence
colors of dye-doped polymer films.
 [Abstract] Yellow, orange, and red luminescence can be achieved by controlling the crystalline polymorphs of 6-cyano HPIP (2).
[Abstract] Yellow, orange, and red luminescence can be achieved by controlling the crystalline polymorphs of 6-cyano HPIP (2).
 [Abstract] Introduction of ‘appropriate’ steric hindrance into 2-(2′-hydroxyphenyl)benzimidazole leads
to polymorphic crystals that exhibit strong (Φ = 0.74) and practically no (Φ = 0.007) ESIPT luminescence.
[Abstract] Introduction of ‘appropriate’ steric hindrance into 2-(2′-hydroxyphenyl)benzimidazole leads
to polymorphic crystals that exhibit strong (Φ = 0.74) and practically no (Φ = 0.007) ESIPT luminescence.
 [Abstract] 2-(2'-Hydroxyphenyl)imidazo[1,2-a]pyridine (HPIP, 1) and its derivatives are synthesized, and
their fluorescence properties are studied. Although all the compounds show faint dual emission
(Φ ≈ 0.01), which is assigned to the normal and excited-state intramolecular proton transfer (ESIPT)
fluorescence in a fluid solution, they generally display efficient ESIPT fluorescence (Φ up to 0.6)
in a polymer matrix. The introduction of electron-donating and electron-withdrawing groups into the phenyl
ring causes blue and red shifts of the ESIPT fluorescence emission band, respectively. On the other hand,
the introduction of such groups into the imidazopyridine part results in fluorescence shifts in the opposite
directions. The results of ab initio quantum chemical calculations of the intramolecular proton-transferred (IPT)
state are well in line with the ESIPT fluorescence energies. The plots of the calculated highest occupied molecular
orbital (HOMO) and lowest unoccupied molecular orbital (LUMO) energy levels against the Hammett substituent constants
(σ) show good linearity with different slopes, which can rationalize the effect of the substituent and its position
on the IPT state. Therefore, we have developed a series of HPIPs as new ESIPT fluorescent compounds and demonstrate
that ESIPT fluorescence properties would be rationally tuned using quantum chemical methods.
[Abstract] 2-(2'-Hydroxyphenyl)imidazo[1,2-a]pyridine (HPIP, 1) and its derivatives are synthesized, and
their fluorescence properties are studied. Although all the compounds show faint dual emission
(Φ ≈ 0.01), which is assigned to the normal and excited-state intramolecular proton transfer (ESIPT)
fluorescence in a fluid solution, they generally display efficient ESIPT fluorescence (Φ up to 0.6)
in a polymer matrix. The introduction of electron-donating and electron-withdrawing groups into the phenyl
ring causes blue and red shifts of the ESIPT fluorescence emission band, respectively. On the other hand,
the introduction of such groups into the imidazopyridine part results in fluorescence shifts in the opposite
directions. The results of ab initio quantum chemical calculations of the intramolecular proton-transferred (IPT)
state are well in line with the ESIPT fluorescence energies. The plots of the calculated highest occupied molecular
orbital (HOMO) and lowest unoccupied molecular orbital (LUMO) energy levels against the Hammett substituent constants
(σ) show good linearity with different slopes, which can rationalize the effect of the substituent and its position
on the IPT state. Therefore, we have developed a series of HPIPs as new ESIPT fluorescent compounds and demonstrate
that ESIPT fluorescence properties would be rationally tuned using quantum chemical methods.
 [Abstract] By using propylamine as a connector, a dimer (L2) and trimer (L3) of 2,2':6',2''-terpyridine (tpy) are
synthesized. They showed the lowest energy π-π* absorption at 354 nm and blue fluorescence at 420 nm
with a moderate quantum yield in solution. Spectral titrations, electrospray ionization mass spectrometry
(ESI-MS), 1 H-1H rotating-frame nuclear Overhauser effect correlation spectroscopy (ROESY), and
structure simulation (MOPAC/PM5) indicated that both L2 and L3 form duplex-type multi-component
complexes with Cd(II), [Cd2(L2)2]4+ and [Cd3(L3)2]6+,
respectively. While the luminescence of these was quite weak in solution (quantum yield Φ < 0.005), the complexes
showed enhanced emission in the solid state with remarkable increase of the quantum yield (Φ = 0.13).
[Abstract] By using propylamine as a connector, a dimer (L2) and trimer (L3) of 2,2':6',2''-terpyridine (tpy) are
synthesized. They showed the lowest energy π-π* absorption at 354 nm and blue fluorescence at 420 nm
with a moderate quantum yield in solution. Spectral titrations, electrospray ionization mass spectrometry
(ESI-MS), 1 H-1H rotating-frame nuclear Overhauser effect correlation spectroscopy (ROESY), and
structure simulation (MOPAC/PM5) indicated that both L2 and L3 form duplex-type multi-component
complexes with Cd(II), [Cd2(L2)2]4+ and [Cd3(L3)2]6+,
respectively. While the luminescence of these was quite weak in solution (quantum yield Φ < 0.005), the complexes
showed enhanced emission in the solid state with remarkable increase of the quantum yield (Φ = 0.13).
 [Abstract] Organic, white luminescent materials were fabricated using a mixture of proton-transfer and
nonproton-transfer fluorophores. 2'-Methoxy and 2'-hydroxy derivatives of 2-phenylimidazo[1,2-a]pyridine
(PIP) have similar UV-absorption properties; however, they exhibit mechanistically different luminescence
respectively ascribable to the normal (~420 nm) and excited-state intramolecular proton transfer processes
(~530 nm) in the solid state. UV-irradiation of mixed solids excites both components concurrently and results
in efficient white luminescence composed of two independent emissions without involving energy transfer process.
White luminescent solids are easily transformed into vapor-deposited films under mild conditions, and
a colorless and transparent thin film by dissolving in PMMA.
[Abstract] Organic, white luminescent materials were fabricated using a mixture of proton-transfer and
nonproton-transfer fluorophores. 2'-Methoxy and 2'-hydroxy derivatives of 2-phenylimidazo[1,2-a]pyridine
(PIP) have similar UV-absorption properties; however, they exhibit mechanistically different luminescence
respectively ascribable to the normal (~420 nm) and excited-state intramolecular proton transfer processes
(~530 nm) in the solid state. UV-irradiation of mixed solids excites both components concurrently and results
in efficient white luminescence composed of two independent emissions without involving energy transfer process.
White luminescent solids are easily transformed into vapor-deposited films under mild conditions, and
a colorless and transparent thin film by dissolving in PMMA.
 Tuning of fluorescence properties of aminoterpyridine fluorophores by N-substitution
Tuning of fluorescence properties of aminoterpyridine fluorophores by N-substitution [Abstract] Several N-alkyl and N-phenyl derivatives of 6-amino- and 6,6-diamino-2,2:6,2-terpyridine
were synthesized, and their fluorescence properties were studied. A successive red-shift
was observed as the number of the N-substituted groups increased. It was also shown that
the susceptivity of the fluorophores to a solvent varied considerably according to the mode
of the N-substitution. While the monoamino-tpys (tpy: 2,2:6,2-terpyridine) suffered almost
complete quenching of their fluorescence in ethanol, the fully N-alkylated diamino-tpys
retained their fluorescence. The results show that N-substitution is a useful way to tune
both the radiation energy and solvent susceptivity of the fluorescence of the amino-tpys.
[Abstract] Several N-alkyl and N-phenyl derivatives of 6-amino- and 6,6-diamino-2,2:6,2-terpyridine
were synthesized, and their fluorescence properties were studied. A successive red-shift
was observed as the number of the N-substituted groups increased. It was also shown that
the susceptivity of the fluorophores to a solvent varied considerably according to the mode
of the N-substitution. While the monoamino-tpys (tpy: 2,2:6,2-terpyridine) suffered almost
complete quenching of their fluorescence in ethanol, the fully N-alkylated diamino-tpys
retained their fluorescence. The results show that N-substitution is a useful way to tune
both the radiation energy and solvent susceptivity of the fluorescence of the amino-tpys.
 [Abstract] The effect of mechanical stress on the solid-state luminescence of 3, a carboxylic acid derivative of
1,3,6,8-tetraphenylpyrene (TPPy), is studied. Grinding the powdery solid of 3 in a mortar results in a
blue shift of luminescence (from yellow to green) instead of the red shift observed for amide (1) and
ester (2) derivatives of TPPy. The initial yellow luminescence is recovered by exposure to solvent
vapour. In contrast, application of compressive stress using an oil press causes practically no change of
the initial yellow luminescence of 3. The yellow luminescence is ascribed to the dimeric form of 3, while
the green luminescence is ascribed to the monomeric state. The macroscale shear stress applied by
grinding induces the dimer-to-monomer transition at the molecular level, which causes the blue shift of
the luminescence. Based on the results, the mechanisms of macroscale compressive and shear stresses to
induce alteration of nanoscale molecular packing and luminescent properties are discussed.
[Abstract] The effect of mechanical stress on the solid-state luminescence of 3, a carboxylic acid derivative of
1,3,6,8-tetraphenylpyrene (TPPy), is studied. Grinding the powdery solid of 3 in a mortar results in a
blue shift of luminescence (from yellow to green) instead of the red shift observed for amide (1) and
ester (2) derivatives of TPPy. The initial yellow luminescence is recovered by exposure to solvent
vapour. In contrast, application of compressive stress using an oil press causes practically no change of
the initial yellow luminescence of 3. The yellow luminescence is ascribed to the dimeric form of 3, while
the green luminescence is ascribed to the monomeric state. The macroscale shear stress applied by
grinding induces the dimer-to-monomer transition at the molecular level, which causes the blue shift of
the luminescence. Based on the results, the mechanisms of macroscale compressive and shear stresses to
induce alteration of nanoscale molecular packing and luminescent properties are discussed. [Abstract] A series of amide and ester derivatives of 1,3,6,8-tetraphenylpyrene (TPPy) were prepared, and
their solid-state luminescence, molecular packing, and piezochromic responses were studied in order to improve
and refine the design concept of piezochromic luminescent materials based on the use of multiple hydrogen bonds
and close packing of disk-shaped aromatic cores as competing factors in the control of molecular packing.
The amide derivative of TPPy having hexyl side chains showed a sensitive luminescence response at a
relatively low applied pressure, which was ascribed to the hydrogen bond-directed H-type columnar packing.
Application of higher pressure induced considerable destruction of the hydrogen bond-directed structure,
leading to the state where close packing was the overriding factor directing the molecular assembly and
showed similar piezochromic response to that of the ester derivative. Based on the results, the role of
the amide hydrogen bonds and the side chains was discussed in detail.
[Abstract] A series of amide and ester derivatives of 1,3,6,8-tetraphenylpyrene (TPPy) were prepared, and
their solid-state luminescence, molecular packing, and piezochromic responses were studied in order to improve
and refine the design concept of piezochromic luminescent materials based on the use of multiple hydrogen bonds
and close packing of disk-shaped aromatic cores as competing factors in the control of molecular packing.
The amide derivative of TPPy having hexyl side chains showed a sensitive luminescence response at a
relatively low applied pressure, which was ascribed to the hydrogen bond-directed H-type columnar packing.
Application of higher pressure induced considerable destruction of the hydrogen bond-directed structure,
leading to the state where close packing was the overriding factor directing the molecular assembly and
showed similar piezochromic response to that of the ester derivative. Based on the results, the role of
the amide hydrogen bonds and the side chains was discussed in detail. Switching of polymorph-dependent ESIPT luminescence of an imidazo[1,2-a]pyridine derivative
Switching of polymorph-dependent ESIPT luminescence of an imidazo[1,2-a]pyridine derivative [Abstract] A novel design principle for materials showing piezochromic luminescence is proposed. Amide-substituted
tetraphenylpyrene C6TPPy, composed of a planar fluorescent core and multiple hydrogen-binding sites,
displays a remarkable change and restoration of its luminescence color by pressing with a spatula and
heating, respectively.
[Abstract] A novel design principle for materials showing piezochromic luminescence is proposed. Amide-substituted
tetraphenylpyrene C6TPPy, composed of a planar fluorescent core and multiple hydrogen-binding sites,
displays a remarkable change and restoration of its luminescence color by pressing with a spatula and
heating, respectively. Reproducible on-off switching of solid-state luminescence by controlling molecular packing through
heat-mode interconversion
Reproducible on-off switching of solid-state luminescence by controlling molecular packing through
heat-mode interconversion