How does crystal gel form?--- Origin of cold rain--- Professor Hajime Tanaka
Institute of Industrial Science
08/1/2017
Researchers at the University of Tokyo, Prof. Hajime Tanaka, Former phD student, Hideyo Tsurusawa, Former Project Research Associate John Russo (currently lecturer at University of Bristol), and a researcher at University of Lyon, Mathieu Leocmach have revealed the elementary kinetic process of crystal-gel formation, i.e., the process in which colloids homogeneously dispersed eventually form a percolated crystal network by interparticle attractions, by 3D real-time confocal microscopy observation.
A colloidal gel is usually in a solid state, where colloidal particles are arrested in a percolated network with a random configuration, but it has been known that, under a certain condition, a gel made of percolated crystal network can be formed. However, it has so far been unclear how such a peculiar gel state is formed and under what conditions.
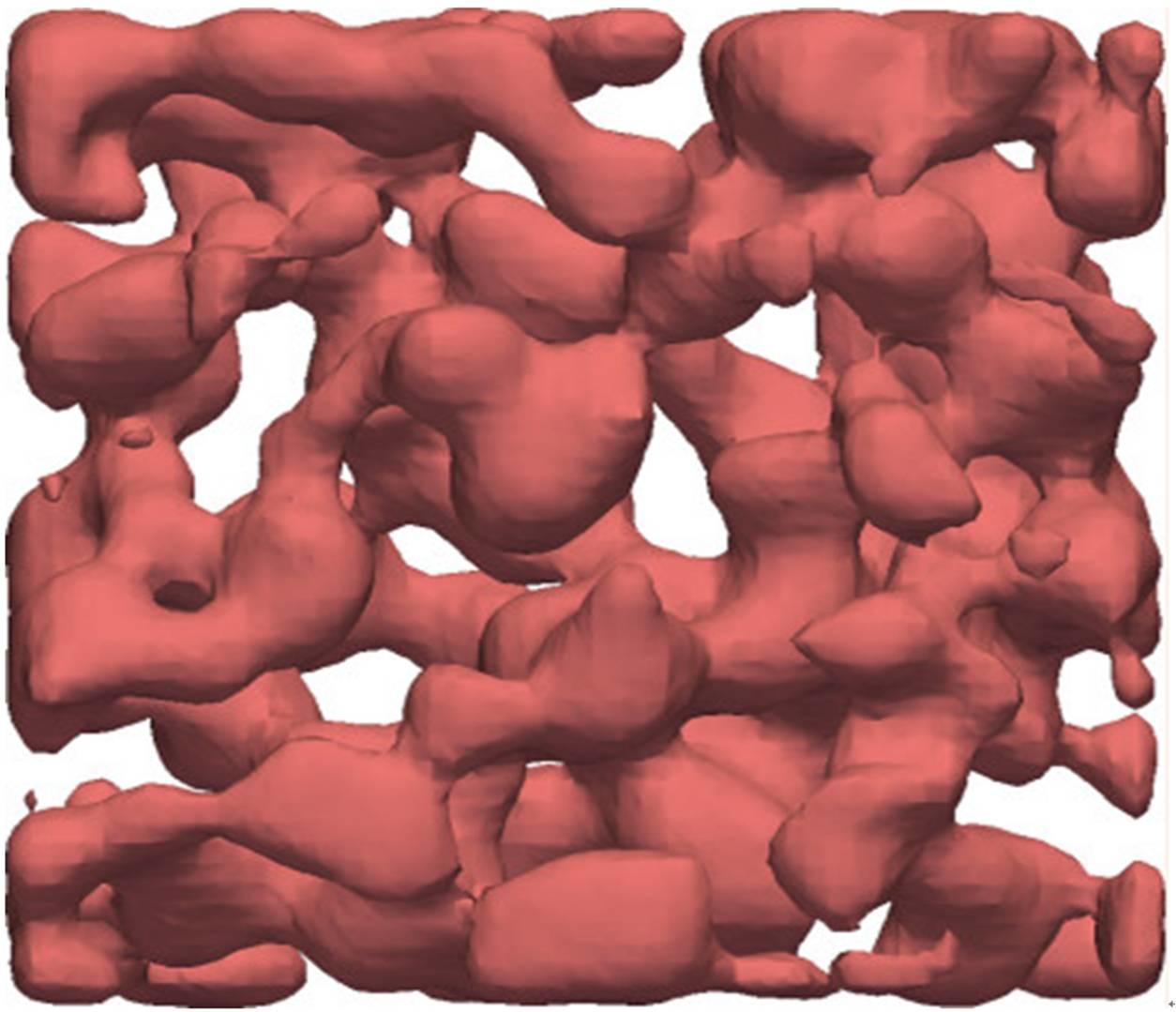
The research group have succeeded in observing the dynamic process of crystal gel formation for the first time, and thus revealed the following elementary process: First, phase separation leads to the formation of a colloid-rich liquid network. Then inside this liquid phase, crystals are nucleated and then grow until they reach the liquid-gas interface. Once crystals are in contact to the gas phase, colloidal particles in the gas phase directly condensate on the crystal surface, since the saturated vapor pressure of the liquid phase is higher than that of the solid phase. This mechanism is found to be the important major process responsible for the growth of crystals. It is actually the same as the so-called Bergeron process, i.e., the ice crystal growth process in mixed phase clouds containing a mixture of supercooled water and ice crystals. Thus, this is regarded as the first example of microscopic observation of the elementary process of cold rain formation. Porous crystals formed in this way have a smooth surface reflecting the formation mechanism and a large surface-volume ratio.
Thu, if such a structure is formed by metallic atoms, it should have a large impact in applications. The research group also provides a physical guidance on what conditions are necessary to realize such a process. So far porous crystals have been formed by a two-step process, phase separation and removal of one of the phases, e.g., by chemical etching. The newly discovered process allows to form porous crystals just by one step. This research promises to form an important part of the design principles behind the development of new porous crystalline materials.
"Thus far, it was unclear how a crystalline gel state is formed. Our study shows that the major mechanism of crystal gel formation is the same as that of the ice crystal formation in mixed phase clouds." says Tanaka. He continues, "I feel that nature is controlled by the law of physics, from the fact that there is a common mechanism behind the apparently unrelated fields, colloidal physics and science of clouds and rain."
Paper:
Hideyo Tsurusawa, John Russo, Mathieu Leocmach, and Hajime Tanaka, "Formation of porous crystals via viscoelastic phase separation", Nature Materials
DOI: 10.1038/NMAT4945
Contact:
Hajime Tanaka, Professor
Tel: +81 3 5452-6125
Fax: +81 3 5452-6126
URL: http://tanakalab.iis.u-tokyo.ac.jp/Top_E.html